What is radioactive decay? – The Hindu

In a random moment, all energy is lost.
| Photo Credit: Unsplash Images
Warning: Danger ahead
Look at the periodic table down below. Other than the blue, all elements depict some amount of radioactivity. The ones though at the bottom? They are the most unstable and display high radioactivity. When it comes to radioactivity, it’s all about the nucleus. Everything depends on it.
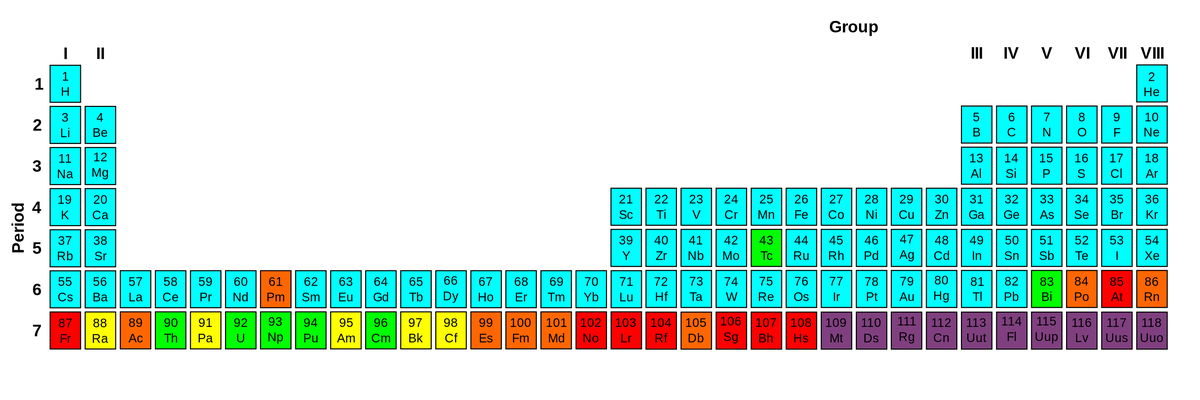
Other than the blue, all elements depict some amount of radioactivity.
| Photo Credit:
Wikimedia Commons
You see, we know radiation is energy. But where do the unstable atoms get this energy? The radiation emitted is because of a process called radioactive decay.
Did you know?
Uranium is a radioactive element with no stable form.
At the site
Unstable nuclei can produce three types of rays or particles each exhibiting different characteristics. In all three cases, the atom undergoes change.
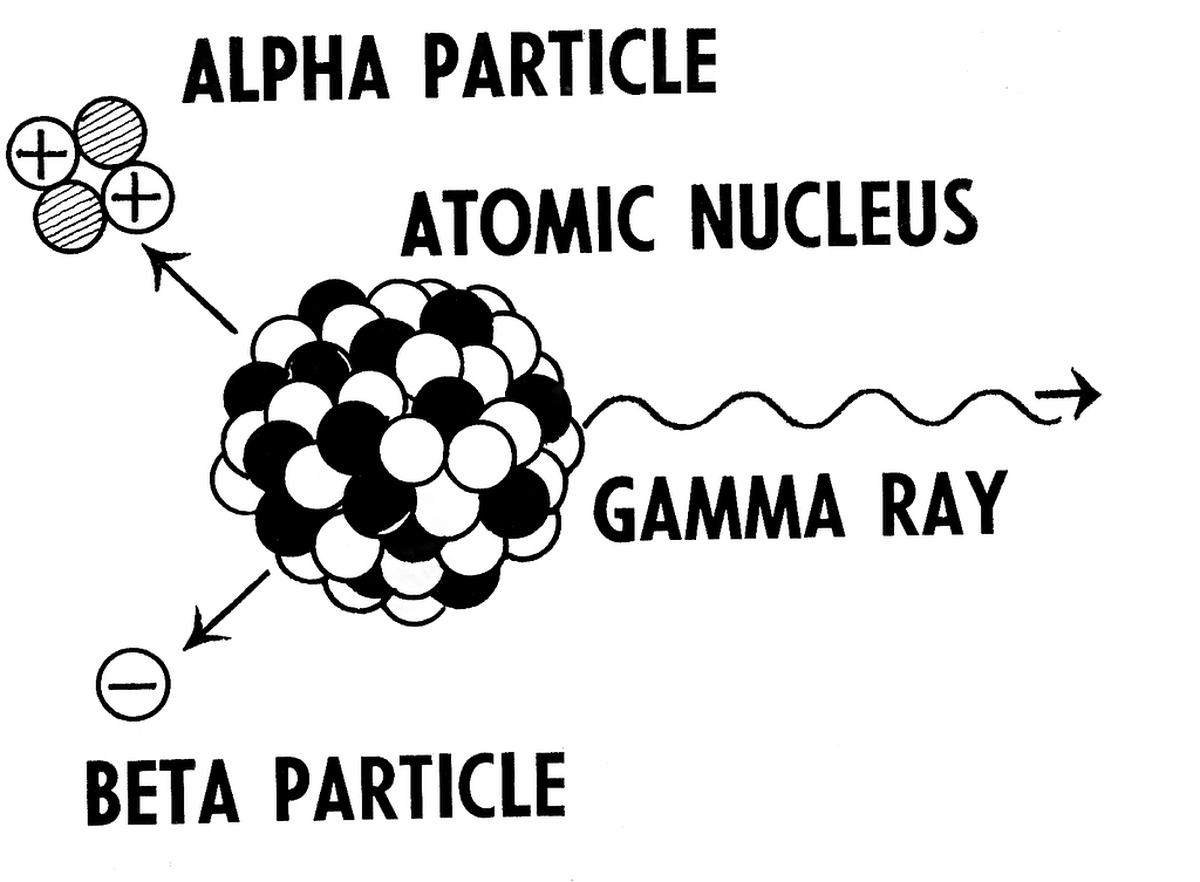
In all three cases, the atom undergoes change.
| Photo Credit:
Picryl
1. Alpha
Alpha particles are positively charged. When the nucleus of the unstable atom has too many protons in its nucleus (like charges repel each other, remember?), it kicks out 2 protons and 2 neutrons (exactly the composition of Helium-4). This is the alpha particle and called alpha decay. When the nucleus ejects out an alpha particle, it becomes lighter and more stable than before.
Eg: Radium (88 protons) undergoes radioactive decay by releasing an alpha particle and becomes Radon (86 protons)
2. Beta
Beta particles are often negatively charged (electron) but sometimes can be positively charged too (positron). A beta particle is essentially a high energy electron that is ejected out of the nucleus. In beta decay, a neutron converts into proton or vice versa and emits a beta particle. Isotopes of Sodium are good examples for this.
3. Gamma
This is a case dealing with excess energy (the atom is very excited). An unstable nucleus emits the excess energy in the form of electromagnetic photons that are highly energetic in nature called gamma rays. Here, protons and neutrons do not change numbers, i.e, atomic number and mass number remain the same. Gamma rays are the same as X-rays except that they are generated by neutrons and not electrons.
Spontaneous overflow?
Yes. While natural radioactivity is random and spontaneous, this process can be induced as well. Just simply alter the composition of the nucleus.
Just simply alter the composition of the nucleus.
| Photo Credit:
Wikimedia Commons
Ionizing Radiation – Radiation that has enough energy that it can forcefully remove the electrons clinging to an atom with all its life. Alpha, Beta, and Gamma particles are all forms of ionizing radiation.
Published – January 26, 2026 04:00 pm IST
Discover more from stock updates now
Subscribe to get the latest posts sent to your email.












