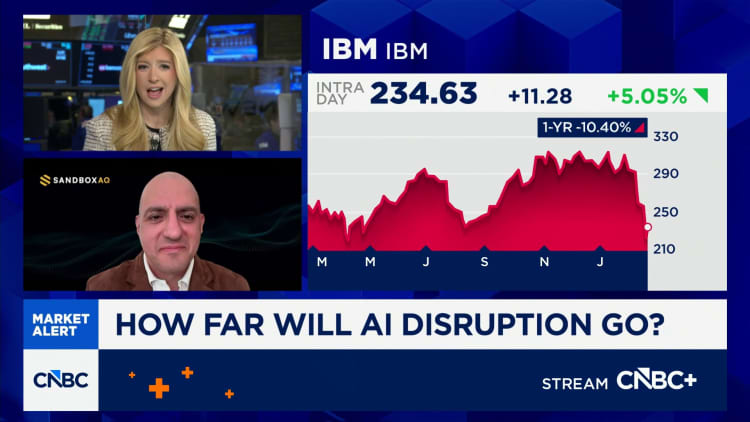
Aurobindo Pharma arm’s biosimilar gets Health Canada NOC

Image used for representational purposes. Photo credits: Special Arrangement
A notice of compliance from Health Canada is issued to a drug manufacturer after a successful review, confirming the product meets regulatory standards for safety, efficacy and quality under the Food and Drug Regulations.
For Dyrupeg, the NOC specifically indicates Health Canada has verified high similarity to an approved reference biologic drug, with no clinically meaningful differences in terms of safety, PK/PD or quality attributes, Aurobindo Pharma said on Friday.
In 2025, Dyrupeg had received marketing authorisation in the European Union from the European Commission and from MHRA, UK. There are three other biosimilar applications from CuraTeQ Biologics seeking marketing authorisation that are currently under review with Health Canada, it said.